Information Center Ozone Water
General Guidelines
Understanding ozone concentration charts can initially become confusing for beginners in ozone applications. We have created a very basic referencing chart specific to ozone exposure times in different volumes of liquids, this should help the user understand ozone charts and specifically select the required minutes of ozonation to achieve estimated dissolved ozone dosages in water.
Typically ozone concentration charts will only indicate the maximum achievable concentration of ozone gas depending on the selected flow rate of oxygen. Extra information is required to calculate saturated ozone in a liquid. We hope this chart helps with exposure times, half life of ozone at room temperatures, basic calculations of saturated ozone in liquids, concentrations and cost effective oxygen usage.
Basic Ozone Concentration Charts (Non-clinical application generators)
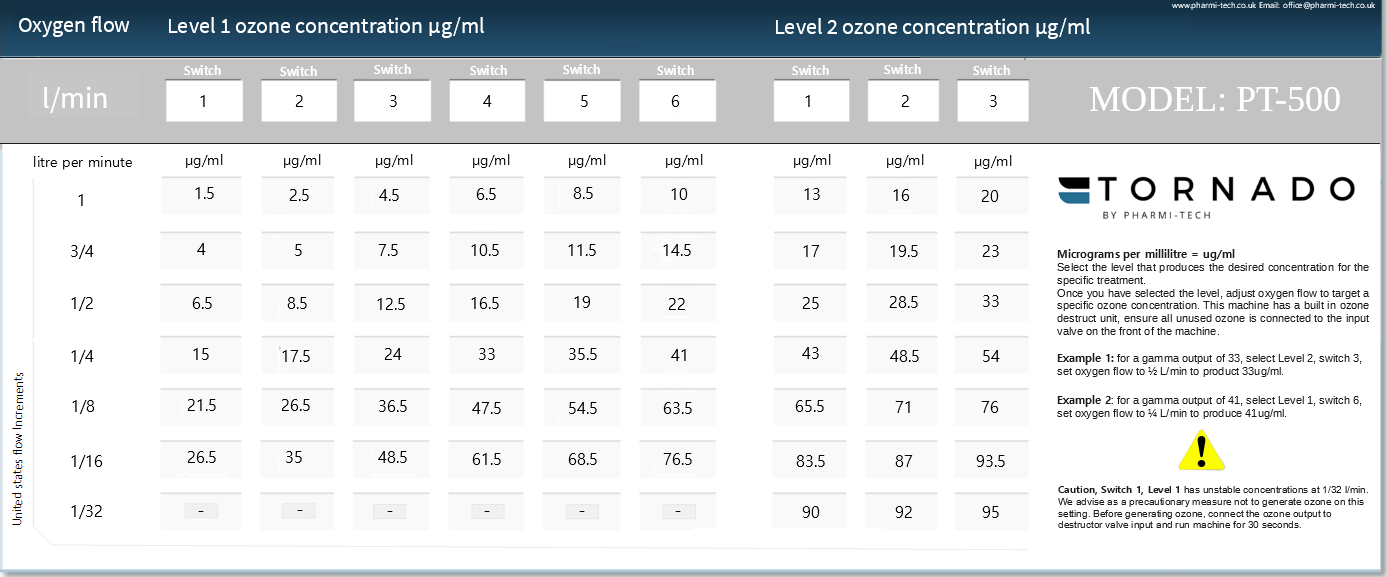
Example chart oxygen flow rate increments are in USA proper fraction geometric sequence. The united kingdom flow rate increment sequence is different from the US sequence, most commonly used and understood flow rates are in US proper fractions. For more information relating to flow rate increments please visit UK flow rates from the information center.
First lets begin with Inorganic and organic liquids.
So ''Physical Absorption'', Physical Absorption refers to the absorption of gas into pure water or some other liquid with which it does not react. Non-chemical absorption is the opposite to chemical abortion, ozone gas is absorbed into organic liquids such as oil's via a chemical reaction called (ozonolysis). So to simplify and re-cap, ozone gas is absorbed into inorganic liquids via ''non-chemical reaction'', ''physical absorption'', and ozone is absorbed into organic liquids via chemical reaction ''chemical absorption''.
Waters ability to absorb light, heat, gases and solids though a non-chemical reaction still remains today a natural phenomenon. It would therefore be logical to conclude the mathematical calculations below are only possible because ozone is absorbed into liquids via non-chemical reaction.
Calculating ozone concentration in organic liquids where they react chemically ''unsaturated oils'' encounter a complicated situation in which the concentrations of the reactants are in general not uniform, so it does not follow the classical Henrys's law in terms of linear solubility variation with pressure. Absorption that takes place simultaneously with the reaction, this organic reaction is called ozonolysis, the chemical reaction effectively converts ''inorganic ozone'' into ''organic ozone'' called ozonides.
Calculation Examples Ozone Gas in ''Inorganic liquids''
Therapeutic ozone is mainly reported in gammas. The conversion for gammas to more familiar units is as follows: 1 ug/ml = 1 mg/l = 1g/m3
= 1 gamma. Gamma is a unit of mass equal to one microgram = 1mcg. There are 1 million micrograms in 1 gram.
Oxygen flow is expressed as a volume of liters per minute (L/min) and the final ozone concentration inversely proportional to the oxygen flow; hence, per time unit, per time unit represents 60 seconds and the higher the oxygen flow the lower the ozone concentration and vice versa.
Example 1: 33ug/ml
of oxygen-ozone gas
Based on the chart above there are seven ozone concentration bands, each band represents a different oxygen flow rate. Ozone Dosages are also referenced in micrograms, and dosages typically range from 1000-10,000mcg. When a dose is expressed as 6000ug, this expression is not a direct reference to concentration, it is a reference indicating the amount of ozone ug/ml
of a specifically selected concentration. For example, If you require dosage of 3300ug, select the required oxygen flow rate, in this scenario the concentration selected is at 33ug/ml, you simply set the oxygen flow to a 1/4 flow and fill a 100ml syringe. A 100ml syringe of oxygen-ozone gas at a concentration of 33ug/ml equates to a dosage of 3300ug. A 50ml measurement equates to a dosage of 1650ug.
Introduction to the ozone half life in solution
Ozone has a half life, which means it will convert back into oxygen over a period of time naturally. Certain environments alter the half life of ozone, the temperature of the water, the pH of the water, the degrees at room temperature, the pressure, and the concentration.
The half life of dissolved ozone in water at room temperature 20-25 °C at a pH 7 (Neutral) is 20 minutes approx. After 20 minutes the saturated ozone will have converted back into oxygen and is no longer effective, a 50% reduction in concentration in a 10 minute period. Depending on the pH of the water and the degrees at room temperature, the half life of ozone ranges from hours to minutes. Using pure distilled water and regulating the temperature at or below 4 degrees, the half life of ozone is approximately 1 hour at a pH 7. Using Double Distilled ''Bi-distilled'' water at room temperature 20-25°C the half life of ozone increases to 1.5 hours. Lowering the temperature of the water below 4 degrees reduces the conductivity and increases the resistance, however bi-distilled water reduces the conductivity and resistance at room temperatures for practicability purposes. It is important to note that the more alkaline the solution the quicker the decomposition. Example: at a pH 8.5 at room temperature the half life of ozone is 18 minutes and at a pH 9.9 the half life is 9 minutes. Ozone is extremely soluble in water, at 25 deg C, ozone solubility is 109mg/l. The solubility of oxygen is 8mg/l. Ozone is 13 times more soluble than oxygen.
Ozone half life in ambient air room temperature
Ozone half life in open Air at (room temperature) can last up to 3 days (72 hours), ozone destruct equipment is essential for all applications. Pilot studies were done relating to half life of ozone gas in ambient air, sealed and contained within its own atmosphere, the results were recorded at a initial starting concentration of 95ug/ml. 72 hours later the ozone gas was still present within the chamber and a result of 14ug/ml was recorded. Ozone half life in air or within its own environment is much different to the ozone half life in a liquid.
Calculation examples: In Solution (pure water)
Generally speaking the solubility is increased by increasing pressure, mass transfer efficiency TE is effected by various factors including bubble size, temperature, pressure, gas/liquid ratio, concentration of ozone. Gas solubility is always limited by the equilibrium between the gas and the saturated solution. The two example expressed below are based on mathematical approximations and estimations with a calculation error of no more than 10%. Furthermore, water has a maximum limitation to the absorption of ozone, continuous bubbling beyond point of saturation
results in an insignificant change in absorption.
Solution of ozone in water takes place according to the law defined by Henry's law, under ideal thermodynamic conditions, the saturation concentration of gas in water is proportional to its concentration. However, this is correct only if the water is absolutely pure (bi-distilled) and the temperature and the ozone pressure remain constant. Bi-distilled water just means water that has been distilled twice.
To calculate ozone concentration in a liquid, you must make calculations based on
(ozone concentration (mcg) x dissolving capability x the volume of liquid).
The concentration of ozone dissolved in a liquid is approximately 0.2, that means at an ozone dosage at 100ug/ml
in 1000ml water volume, the following amount of ozone will be dissolved (20%). 20% = 0.2 x 100 = 20mcg or 20ug of ozone. (Calculation error no more than 10%).
Example 1: low Concentration setting : 33ug/ml (250ml water) 20%
Minutes of infusing though pure water (bi-distilled) at 33ug/ml (250ml container). 4 minutes = 1 liter of oxygen.
1/4 flow = 1÷4= 0.25 x 33 = 8.25 x 4 = 33 (4 minutes = 1 cycle)
As stated previously under (calculation ozone gas section one) oxygen flow
regulators represents a unit measure of time, in this case we selected a 1/4 flow, 1/4 represents 60 seconds, and produces 250cc of oxygen per minute. This means the ozone generator is producing 8250 micrograms per
minute, and takes 4
minutes to complete a liter of oxygen, or one cycle. 8250 x 4 = 33,000.
Dose (mcg) = 20% x concentration 03 x Volume of water (ml)
6.6 x 33 x 0.25 = Dosage 54 mcg. between 6-7ug/ml 03 concentration.
Example 2: Higher concentration 76ug/ml (500ml water) 20%
Minutes of bubbling through pure water (bi-distilled) at 76ug/ml. 8 minutes = 1 liter of oxygen.
1/8 flow = 1÷8 = 0.125 x 76 = 9500 x 8 =76 (8 minutes = 1 cycle)
In this scenario we selected oxygen flow rate of 1/8 to produce a ozone concentration of 76ug/ml. The ozone generator produces 950 micrograms per minute. As a slower flow rate was selected to produce a higher concentration of ozone, the measure of time per Minuit also changed. It now takes 8 minutes to complete a full cycle.
Dose (mcg) = 20% x Concentration 03 x Volume of water (ml)
Calculation : 76 x 02 = 15.2 ug/ml.
Second calculation dosage: 15.2 x 76 x 0.5 = 577.6mcg.
Saturation time (the interesting part)
Now you may be a little overwhelmed with all those previous calculations in different volumes of liquid and adjusted pressures, the interesting part is as long as the water is absolutely pure, and the saturation concentration of gas in water is proportional its concentration, and the pressure and temperature remain a constant, ozone saturation can be reached within 6-8 minutes regardless of water volume. Continuous bubbling past 6-8 minutes has no significant change in dissolved ozone, as long as the variables are a constant and ozone is passed though as a column of gas at a constant rate and constant pressure, volume of water has no influence on saturation time. Dr. Velio Bocci demonstrated this in his experiments.
So there are many natural variables to consider when it comes to ozone saturation in a liquid, and of course the purity of the water, the natural variables influencing the transfer efficiency,TE are factors including but not limited to bubble size, temperature, pressure, gas/liquid ratio, concentration of ozone, pH etc..........
however keeping all variables constant in a controlled environment can overcome these external influences.
As water will only retain 20-28% of the ozone concentration, ozonating water on a maximum setting of 100ug/ml will retain only 20-28ug/ml after 6-8 minutes, this includes dosage limitation.
Generally speaking ozonating time is extended beyond the point of saturation to complete a cycle relevant to the selected flow rate, this may last from 8 to 32 minutes. When ozonating water in the correct conditions and equipment suitable for overcoming external influences, selecting a high ozone concentration between 70-90ug/ml and ozonating for 6-8 minutes will produce adequate saturation at concentrations ranging between 15-25 ug/ml, regardless of liquid volume and all mathematical equations are no longer required.
Example:






